" Pure-white, stone-cold fiber optic lighting sytems "
How Photons Meet Atoms
Light has mass. Photons are particles. They travel through the wide spaces between atoms.
But when a photon gets so close that it interferes with the electron rings of an atom, the photon is either attracted into the atom or is repelled as it squeezes the atom.
The atom is excited. Physicists say its energy state rises as its orbits are not acting in its normal “rest” state.
Matter that is excited can change.
If the photon’s diameter matches an orbit ring, the atom’s electrons in orbit gimbal, repel the intruder and the photon bounces out of the material never changing its light speed.
The encounter is defined as reflected energy matching. The photon can cause sight.
But if the photon is broken apart, the molecule that stopped its flight path receives two extra electrons.
The electron cloud becomes more crowded. The photon’s electron tries to scramble into the rings of the atom.
The photon’s positron is buffeted and agitated into reorienting back into a normal electron spin, shifting from a positive charge into a negative charge.
The photon is gone. Scientists use the word “annihilated”. But the photon is really broken apart leaving two free electrons in the material.
This is familiar territory. This is high school chemistry.
Enough extra electrons and a molecule’s atoms will stabilize without sharing electrons or having the bonding attractions with its neighbors.
The bigger molecule breaks into two smaller molecules. The chemistry changes.
This is photochemical damage…usually in a bad way.
This is also familiar territory for biology. The retina in the eye secretes enzymes with fragile bonds that are susceptible to photons from 380 nm to 770 nm.
When the photon breaks apart, an enzyme molecule is excited.
Testing shows six photons will generate data that a human “sees”.
Since the reactions are tied to quantum electrodynamics, no one knows if: 1) it is six photons that are needed to break a single enzyme
into releasing electrons for the nerve to transmit to the brain; or 2) if the six photons break apart six enzymes and the six enzymes are added together to produce the signal.
However, this is photochemical damage…usually in a good way. The brain “sees” the photon as data.
Most everyone remembers chemistry class and using a Bunsen burner. Raise the temperature by 10°C. The chemical reaction doubles.
Light in a museum gallery or in the home of an important collector does the same thing. Photons break apart. Extra electrons are added.
The object’s chemistry changes. Important things fade, crack, yellow, brown, blur, bloom, embrittle, splinter, fray, shred, collapse,
crosslink…the changes are seen in different forms. But the object is never the same. It hasn’t the presentation it use to have.
So It also looses monetary valuation even if the purchase price rises as the damage stifled the full capital gains. It can even distort history.
The photon(s) created enough quantum energy for a chemical change. This is the heart of photochemical damage. It is also an example of the Second Law of Thermodynamics.
(To simplify, the Second Law of Thermodynamics is entropy. Light brings in particles or energy that excites the atoms in molecules and the molecules break into smaller molecules. Light turns big molecules into smaller molecules. Things entropy and fade, yellow, embrittle, corrode, change, weaken, crack, fall apart, crosslink, oxidize…and since there is really no place on the planet without some form of light, remember photons are far more than visible light, but all radiation including cosmic, objects age and break down. That is not a bad thing. It is one of the ways the earth cleans itself by using photochemical change created by the sun to get rid of things.)
To repeat, Dr. Richard Feynman stated, “I want to emphasize that light comes in this form - particles.
It is very important to know that light behaves like particles, especially for those of you who have gone to school,
where you were probably told something about light behaving like waves. I’m telling you the way it does behave - like particles…light is made of particles.” (Feynman, QED, 1985, pg 15.)
Feynman also stated, “If, in some cataclysm, all scientific knowledge were to be destroyed, and only
one sentence passed on to the next generation of creatures, what statement would contain the most information
in the fewest words? I believe it is the atomic hypothesis (or the atomic fact, or whatever you wish to call it)
that all things are made of atoms - little particles that move around in perpetual motion,
attracting each other when they are a little distance apart, but repelling when squeezed into one another.”
Photons Are Part of That Elegant “Atomic Hypothesis”
Photons are little particles.
These particles were once moving around in a circle in a nucleus with
all those other particles in perpetual motion or jumping from atom to atom in a stream of electricity.
But crowding caused an electron to flip backwards into a positive charge.
Then two of these atomic particles out of the cloud are attracted to each other. The conversion orbit usually determines the wavelength. It is orbital mechanics.
The atomic orbit is translated into spinning around as a pair get ejected into space.
There is a lot of energy in motion in these atomic orbits. That energy gets configured into a wave motion
as the electron and positron rotate around each other. More of that energy is identified as forward literal
light speed motion as the photon zooms away from where it was born.
It is a light ray. It is a wave made of particles. It is a “corpuscle” of light. It is a photon.
For safely displaying a collection, the photon’s ability to impact other particles is critical.
The two particles are “energy”, but really the pair is a thing. After traveling through lots and lots of space,
probability finally makes the pair encounter another atom with all its perpetual moving particles.
“We know that light is made of particles, because we can take a very sensitive instrument that makes
clicks when light shines on it, and if the light gets dimmer, the clicks remain just as loud -
there are fewer of them. Thus light is something like raindrops - each little lump of light is called a photon -
and if the light is all the same color, all the raindrops are the same size…I want to emphasize that light comes in this form - particles…light is made of particles.”
Feynman further explained the spin of the photon as “..the stopwatch hand (measuring spin velocity)
turns around faster when it times a blue photon than it does a red photon. In fact that is the only
difference between a red photon and a blue photon (or a photon of any other color, including radio waves,
x-rays, and so on) - the speed of the stopwatch hand.”
Basically there are more spins over the same forward movement of the electron and positron in a blue
wavelength compared to a red. Or more revolutions in a UV wavelength with a smaller spin diameter compared to an IR wavelength.
But all wavelengths are the same paired system.
Atoms are mostly space. These particles are widely separated by nothing. Isaac Asimov in the book, Atom, wrote,
“…the nucleus has anywhere from 99.945 to 99.975 percent of the mass of an atom…the nucleus is tiny in size, with a diameter only 1/100,000 that of the atom.”
Or in other words, the electrons and space make up 99,999/100,000 of an atom’s size.
And though the common symbol for an atom is neat with three perfect little ovals centered on a dot,
the electron rings are a long distance from the nucleus and there with all sorts of orbits.
In reality, atoms are magnificent in order and control. But they look horribly messy.
Atoms are not necessarily even round, but usually more shaped like solar systems with extra orbits outside the main plane.
And they can be fat or tilted or even defective with missing parts.
So not only is there abundant space, but there is orientation.
Even the photon itself has lots of space with nothing in it. Molecules are not solid at all.
Neither are photons. A typical photon will travel through over 80,000 layers of gold molecules before being influenced by any atom in the structure.
Photons rotate messily too. Feynman states that photons rotate in certain kinds of spins: right open,
left open or screwy. But the more science explores particles, the discoveries find even bizarre permutations.
For example there is the odd “postronium” that is an electron rotating around the positron as if the positron is stationary,
sort of like a hydrogen atom, with a very quick decay that spins into a moving photon.
What to Know about Photons
The point is that photons move perfectly straight through space.
The wavelength is a measured distance of a single rotation of the photon as it travels.
The wavelength is the distance between the electron and the positron.
But photons spin and tumble in different ways as they travel. Each photon is consistent in
its tumble unless it is disturbed by brushing against some other particles. And there is variety.
But every one of the photons has a wave-like travel.
That orientation in variety impacts how photons interact with matter.
Since the atoms have variety in orientation too, encounters produce interesting results.
This makes matching and mismatching such complex mathematics that quantum physicists use diagrams to describe the quantum electrodynamics.
Drawings make it easier to understand than the supporting math.
For museum needs, photons are moving particles that can irreparably alter a rare object.
It is all based on probability. More light equals more possible photochemical damage. This is the heart of the recommended footcandle requirements established in museums worldwide.
Set footcandle limits lower the probability of chemical change by limiting the exposure.
Keep the numbers of photons high enough people can see well if they are light adapted.
But make the number of photons low enough to extend the life of the artifact.
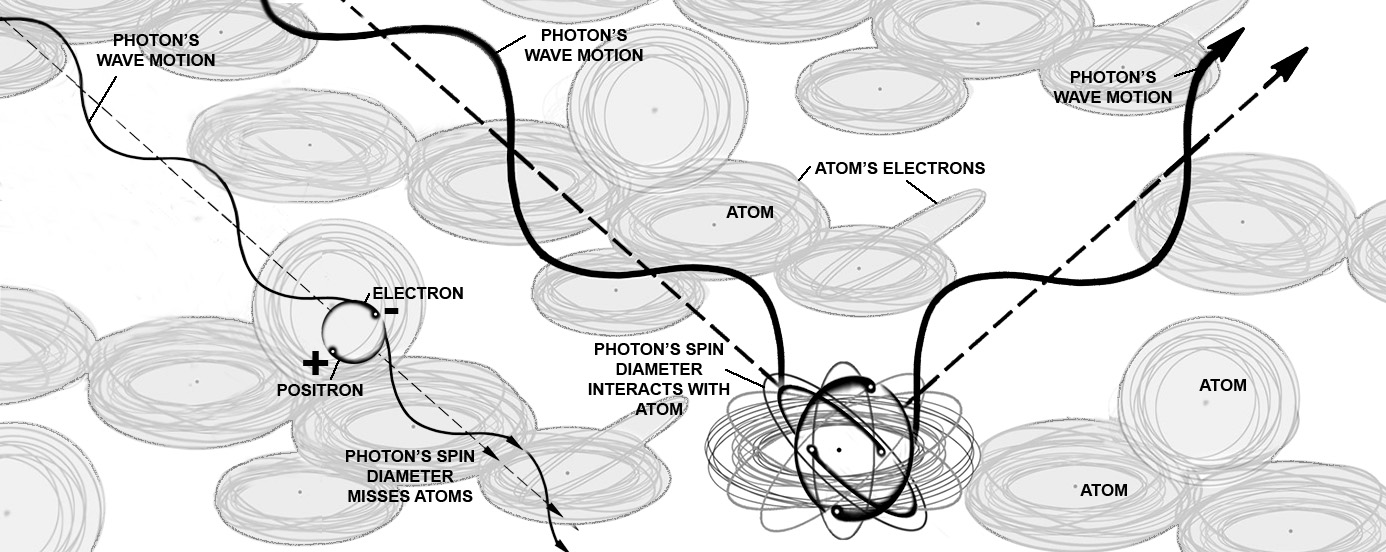
If the photon’s distance between the electron and the positron along with the photon’s leading edge of its
tumbling spin match any orbit of an electron in the cloud of an atom, the electron in the photon tries to move towards the positive attraction of the
nucleus and crowd away for the other electrons. The positron is linked to the electron. So it gets dragged in wanting to reflect back out of the
atom as it is repelled by the nucleus’ positive charge.
The photon excites the atom. The electron’s negative charge makes the atom’s electrons swerve, avoid and repel tangling the normal orbits.
The positron’s positive attraction confuses the electron rings of the atom causing gimbaling and further distrubing ordered orbits, but jumbled and seeking stability.
The energy level of the atom rises. The electron rings try to accept half the photon and yet avoid the other half.
The electrons in the atom’s cloud dance. The orbits get messier. And the jerking motion as forces wrench at the photon makes events happen.
One of Four Events Happen
1. NOTHING HAPPENS. The match can be poor enough that the photon keeps traveling with just a little change in tumble and
settles back down at light speed on its original ray’s path. The photon’s passing near may excite the atom, raising its energy level (activity).
The electron cloud could grow a little. But it is a fast event.
The atom quickly stabilizes back to its pre-photon state. The chemistry stays the same. The molecule does not change.
2. REFLECTED LIGHT, POTENTIAL SIGHT.
The photon’s spin matches the orbit of an electron ring in the atom and gimbals the photon against a specific matching electron ring.
The atom reflects the photon.
Usually the whole photon reverses back out of the material.
The photon cannot crowd into the other electrons.
It cannot wedge itself into the host of electrons.
The electrons’ negative charges spit the neutrally charged whole photon out as the photon found no room to crowd into the atom.
The photon stays assembled.
This is reflected data. These are the photons humans see. This is useful knowledge.
The more photons that match an object, the more people see.
Better sight means more enjoyment and faster learning. This is Reflected Energy Matching Theory.
The atom stabilizes back to the energy level it was before the encounter.
The chemistry stays the same. The molecule does not change.
3. DAMAGE CAUSED BY LIGHT.
Probability causes the photon to encounter the electron rings of an atom that are mismatched to the photon’s spin.
None of the electron orbits of the atom match the diameter or orientation of the photon enough to gimbal or act as a whole to repel the photon.
Instead the photon wedges itself into the electron cloud.
The photon’s electron causes other electrons to speed or slow which subjects the positron to being buffeted.
The encounters get wild as the positron keeps trying to escape the atoms positive influence.
Yet it is pulled by electrons crowding as the positron is attracted to the opposite charges.
Suddenly one encounter is just too close.
The positron wrenches into a reverse spin as it tries to either avoid or latch onto another negative electron in the cloud.
The positron’s spin reverses. It turns back into an electron.
The photon was squeezed into the atom with the pair coming apart. The new electrons agitate the original electrons.
The atom has two extra electrons. Those electrons might stay. Or they might migrate.
Photochemical Damage. Two new electrons can be enough to stabilize the single atom to stand more on its own without sharing electrons or a charge with its neighboring atom(s) as a bond.
The outer ring (the one that controls much of the chemistry) is different. Valence electrons have been added.
Sometimes the crowding happens closer to the nucleus. But spare electrons from either the original photon or
from the atom’s original cloud of electrons are shoved to the outside into the valence ring.
The atom rearranges itself. Quantum electrodynamics theory says the atom will expend as little energy as possible to re-stabilize. But the atom changes.
The atom drops its link. The chemistry has changed. The single molecule is now two smaller molecules.
Sometimes the agitation is such that the extra electrons will twist the atom around and though it has dropped its link to its neighbor, it finds equilibrium in a stray atom that wasn’t part of the original chemistry. Again, the atom will re-stabilize itself with the least effort or loss in energy. The atom bonds with something going by like oxygen, acid or a form of pollution. All these chemical changes are photochemical damage.
(The book, Quantum Mechanics for Applied Physics and Engineering, by Fromhold, pg 132, puts it this way. “The chemical properties of different atoms for element(s) are determined principally by the uppermost filled energy levels, since those higher energy electrons, being less tightly bound to the atomic core most easily share themselves with other atomic cores for the formation of chemical bonds in molecules and solids.” The photon’s annihilation has added electrons to the uppermost and higher energy level of the atom. Or, in other words, add to the more active, in energy, valence ring.)
The key is the mismatch. It created the change. If the light had better matched the object, the atom would have reflected the photon.
Reflected photons do not loose spare electrons into the atomic structure. But absorbed light does. Absorbed light causes harm. And absorbed light never generates sight.
Photomechanical Damage. If the bonds are strong enough, the atom will hold to its neighboring atom even with the added energy.
The atom is more active. There is more chance for some other photon to produce chemical change. But the molecule does not break apart.
However, two additional electrons have been added to the chemistry. There is a change. The atom has grown.
And often the atom will want to radiate that extra electron pair back out. But it can’t, because its neighbors are just as active in getting hit by photons.
The atom literally grows as more room is needed for the electron cloud. Again, particles are squeezed. But the whole surface shuffles.
The object’s outside surface gets bigger.
The atoms that are much, much deeper inside the material are not excited. The photons are not reaching clear into the object’s
depth unless they have a very tight spin diameter. So the inside of the object may slowly warm and grow. But it is at a different rate.
It is the outside layers that are agitated, crowding and using more volume.
The molecules are excited, but stay together. They are chemically stable. But the mechanical growth is different.
This isn’t so much quanta any more as it is basic physics. Warmer things spread molecules out more. The atoms use more room.
Cooler things let molecules rest in tighter space. When there is a temperature difference in surfaces, the material can sheer or break.
It is not the individual chemistry that ha changed, but the surfaces and interfaces have physically sheered.
The photons effect the outer rings. When the photons stop coming, the atoms radiate electrons out of their agitated outer valence
rings as photons from those larger orbits. The photons with larger spin diameters are IR. The surface can get rid of heat faster than the interior of the object.
In cooling, the artifact mechanically works forces against each other. Turning off the lights has caused the cycle to reverse. The surface is tighter than the inside.
Again, over time, the piece breaks or cracks.
These expanding and contracting changes, all mechanical and much more basic than looking at the atomic level’s chemistry, happen day after day.
Over time, they physically stress the artifact. Over time, things crack, split, check, spall, shred…all caused by photons.
Photons physically warmed and cooled the object at different rates. It broke the object. It is mechanical damage. And it was growing,
contracting, growing, contracting, growing, contracting every day the lights were turned on and off.
But there is a fourth event that can happen.
4. A PHOTOCHEMICAL SURPRISE. Because part of this discussion is to understand light sources, it is a need-to-know fact that
some chemicals have mostly stabile rings with certain unstable rings inside the atom. In chemistry the focus is on the valence
ring or the outer shell of the cloud of electrons. But in dealing with photons, the spin diameters create interesting results as a photon can
destabilize an atom in rings other than the valence ring. It can get inside the cloud which is why there are some very odd explanations
given as to how light sources actually work.
A photon’s spin diameter can match an inner orbit of an atom. The outside valiance ring will respond. But the change happens inside the orbits closer to the nucleus.
The photon crams into an inner ring.
The electron cloud becomes more agitated.
The photon breaks apart adding its electron and positron turned electron into that inner ring.
Electrons start shifting. Another ring gets too crowded and spits out a photon.
This phenomenon is fluorescence.
A photon is absorbed just like the description of photochemical damage.
But the atom’s cloud shuffles the two spare electrons to a specific ring.
That ring’s orbit flips out an electron and positron with the diameter spin of a certain wavelength.
The atom does not keep the two spare electrons, because it spits out a photon.
The entering photon can be a variety of wavelengths. But it is absorbed.
The exiting photon is determined by the way the atom’s cloud is constructed and its orbits.
Since one orbit is prone to react to entering photons, roughly the same diameter comes out over and over again.
As yet, it is debated rather the photon is truly absorbed and a new pair created or if the original photon is shuffled and adjusted to a different wavelength.
NoUVIR believes the entering photon is pulled into the orbits and a new photon is created from the unstable ring.
The take-away information is to know that one type of photon goes in. And another photon with a different wavelength comes out.
If the right materials are selected, often the photon coming out has the same wavelength over and over again.
Because orbits and clouds are messy, the wavelength varies slightly. But the fluorescence is in a general band,
like UV around 250 nm or visible green at 560 nm. Again, the point is that one wavelength of light goes in and another wavelength of light comes out.
But the one that comes out is controlled by the chemistry of the choice of materials.
It has to do with the orbital mechanics of the atom’s rings. These clouds of electrons travel around the nucleus in different tracks.
Again, the orbits are messy. But there is order.
And all of the electrons have mass. It is not a lot of mass. But on a minute scale, it is still a form of orbital mechanics like a solar system.
The photon has mass too. Again, it is a tiny fraction of the huge volume of mass contained in the nucleus. But gravity influences the mass.
Filament lamps use electricity to generate photons from the outer ring of the atom. As more and more electrons rush in,
the filament’s atoms create more and more photons closer to the nucleus. The light source marches from IR into the visible wavelengths.
Phosphor lamps instead convert the electricity into a specific wavelength, often UV or blue, and then convert those photons with phosphors that fluoresce.
Fluorescent lamps are not the only phosphor light sources.
Light Emitting Diodes, LEDs, use special chemistry to make blue visible light, then convert it to “white” light.
The instability of an atom’s electron ring converts the electrons through a diode into spinning backward positrons from a specific orbit that establishes the wavelength,
then the phosphors convert the wavelength into more useable visible light. That material that converts is the “emitting diode” orbiting out photons through
a hole with near identical wavelengths, because of the structure. Some LEDs stop here without adding phosphors.
But many then convert some of those wavelengths to other visible wavelengths using fluorescence through a coating.
Click HERE for Article "Don' Get LED Down the Garden Path" pdf
Click HERE for Article "Museum Lighitng: Pure and Simple" pdf
Simplified Quantum Electrodynamics
It is all about what photons are reflected as data and what photons are absorbed. Reflected light does not cause damage. Reflected light does not create chemical change.
Absorbed light does. The more light absorbed, the more damaging the source becomes. It is always mismatched photons that enter atoms and break up the molecules.
The photons come apart and add electrons to the chemistry. The molecules drop atomic bonds. The chemical change can be seen as different photons are now reflected.
We say the object has faded.
A museum conservator says it has been damaged.
An auctioneer says the object has “condition” issues, so it is worth less.
Different light sources make photons in different ways. Different light sources have different damage rates.
It is all about the photons the light source creates when it converts electricity.
Knowing how each light works and what the light output is lets light sources be matched to the objects they are lighting.
Can light sources be ranked? Yes.
Have they been tested for damage? Yes.
Want to see peer-reviewed test results, not just words? See the button LIGHTING SCIENCE FROM FOSSILS TO SPACECRAFT on the home page. Also click below for more quantum physics.
FOR UNIVERSITY STUDENTS: This web site is for people who need to protect our national heritage from light damage, often art majors. But in universities, nomenclature is really important. That means that you need to use big, technical words in any university setting. Dr. Feynman could get away with lecturing in a very loose and understandable style. A student often cannot. Speak in scientific words. Write using exacting scientific terms.
REFERENCES: See the pdf files for published papers with formal documentation of quotes, references, source materials for further reading and test procedures. Look at the top of the home page at PEER-REVIEWED SCIENCE.